1 sec package-insert challenge: NO vaccine was tested on a long-term placebo-controlled trial
It only takes 1 second to look at the 2 critical red columns in the table!
How could anyone declare that vaccines are “safe and effective” if they were never properly tested?
Challenge them to follow the science and actually READ the package inserts!
Systematically violating the right to informed consent, the medical system never informs and explains, the risks in each section of the package insert:
Section 1: Indications and Usage - The FDA-approved uses for the drug.
Section 2: Dosage and Administration - Recommended dosage, administration instructions, and any necessary modifications.
Section 3: Dosage Forms and Strengths - A description of the physical appearance, form, and strength of the available medication.
Section 4: Contraindications - Situations and conditions where the drug should not be used due to a high risk of harm.
Section 5: Warnings and Precautions - Information on serious or clinically significant potential adverse reactions and safety hazards.
Section 6: Adverse Reactions - A list of all side effects observed during clinical trials and postmarketing experience.
Section 7: Drug Interactions - Clinically important interactions with other drugs, food, or products.
Section 8: Use in Specific Populations - Information regarding use in pregnancy, lactation, pediatric/geriatric patients, or those with organ impairment.
Section 9: Drug Abuse and Dependence - Information on the drug’s potential for abuse, misuse, and physical or psychological dependence.
Section 10: Overdosage - Expected signs and symptoms of an overdose and recommended treatment.
Section 11: Description - The chemical name, structural formula, and a list of active and inactive ingredients.
Section 12: Clinical Pharmacology - Details on how the drug works (mechanism of action), pharmacodynamics, and pharmacokinetics.
Section 13: Nonclinical Toxicology - Information from animal studies, such as potential for cancer or effects on fertility.
Section 14: Clinical Studies - A summary of the studies that established the drug’s safety and effectiveness.
Section 15: References - A list of scientific references if applicable.
Section 16: How Supplied/Storage and Handling - The available package forms, identifying features (color, shape), and storage instructions.
Section 17: Patient Counseling Information - Key information for healthcare providers to discuss with patients, and a reference to any FDA-approved patient labeling (e.g., a Medication Guide).
Those sections aren’t there to fill up an unreadable insert, but because each one is very important in order to evaluate the risks, and compare them to the potential benefits.
If the doctor never read the Full Prescribing Information (FPI), they are rationally incapable of prescribing them.
https://www.fda.gov/about-fda/oncology-center-excellence/how-do-i-use-prescription-drug-labeling
Important note
Even by reading the package inserts, it’s impossible to reach an objective cost-benefit analysis based on the inserts alone, because the information is generated by the manufacturer, which runs the clinical trials and has severe conflicts of interest.
Even more, if we consider that any vaccine vial could be different from another, since no government really analyzes them. No standarization, no science. No uniformity, no “safe and effective”.
1 image saves 1000 words
It takes 1 second to look at the 2 critical red columns:
Pdf with zoom-in table and links:
Important note
Nobody ever controls if the fake non-saline “placebos” were tainted so that the adverse reactions to vaccines would appear normal, compared to the nocebos: so-called placebos causing harm, like those containing neurotoxins (aluminum, mercury, etc.).
That is a plausible hypothesis, especially if we recall that nobody controlled if the ivermectin pills were not tainted or really had the informed dose in the very few studies “proving” that it was useless against COVID: https://c19early.org/i
Pdf Transcription
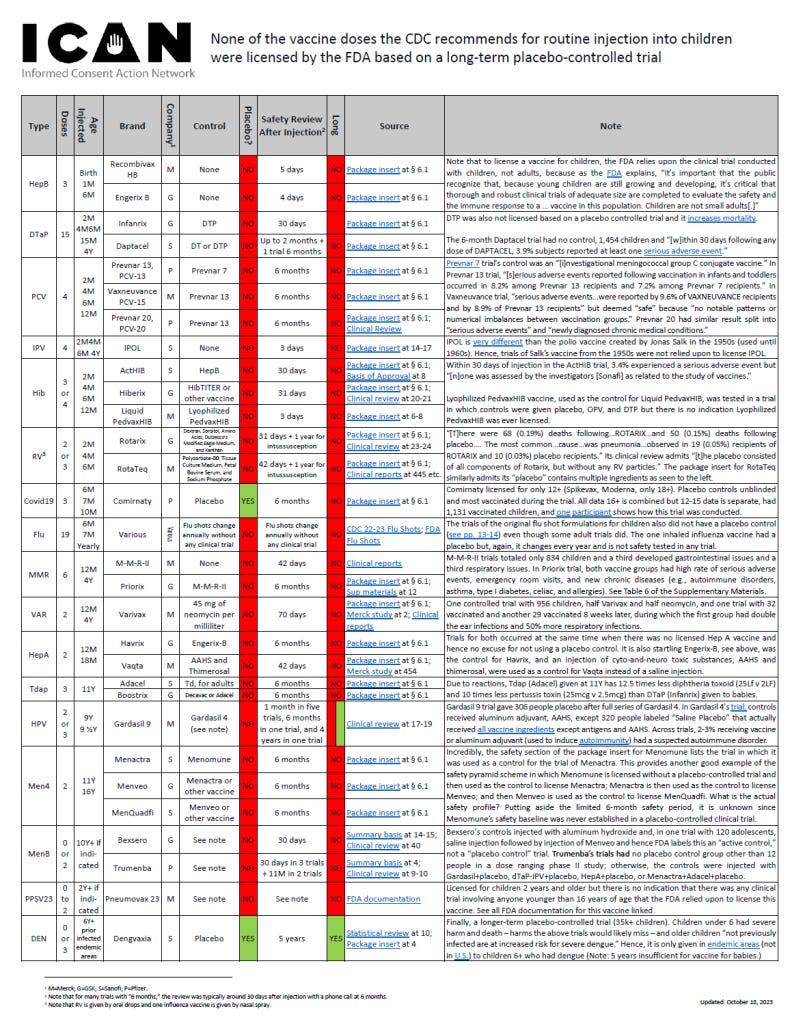
None of the Vaccine Doses the CDC Recommends for Routine Injection into Children Were Licensed by the FDA Based on a Long-Term Placebo-Controlled Trial
HepB
Doses: 3
Age: Birth 1M 6M
Recombivax HB
Manufacturer: Merck
Control: None
Placebo?: NO
Safety Review After Injection: 5 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74274/download
Note: Note that to license a vaccine for children, the FDA relies upon the clinical trial conducted with children, not adults, because as the FDA explains, “ It’s important that the public recognize that, because young children are still growing and developing, it’s critical that thorough and robust clinical trials of adequate size are completed to evaluate the safety and the immune response to a … vaccine in t his population. Children are not small adults [.] ”
Engerix B
Manufacturer: GlaxoSmithKline
Control: None
Placebo?: NO
Safety Review After Injection: 4 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/119403/download
DTaP
Doses: 15
Age: 2M 4M6M 15 M 4Y
Infanrix
Manufacturer: GlaxoSmithKline
Control: DTP
Placebo?: NO
Safety Review After Injection: 30 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75157/download
Note: DTP was also not licensed based on a placebo controlled trial and it increases mortality . The 6 - month Daptacel trial had no control, 1,454 children and “[w]ithin 30 days f ollowing any dose of DAPTACEL, 3.9% subjects reported at least one serious adverse event .”
Daptacel
Manufacturer: Sanofi
Control: DT or DTP
Placebo?: NO
Safety Review After Injection: Up to 2 months + 1 trial 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74035/download
PCV
Doses: 4
Age: 2M 4M 6M 12M
Prevnar 13, PCV - 13
Manufacturer: Pfizer
Control: Prevnar 7
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/files/vaccines%2C%2520blood%2520&%2520biologics/published/Package-Insert------Prevnar-13.pdf
Note: Prevnar 7 trial’s control was an “ [i] nvestigational meningococcal group C conjugate vaccine.” In Prevnar 13 trial, “ [s] erious adverse events reported following vaccination in infants and toddlers occurred in 8.2% among Prevnar 1 3 recipients and 7.2% among Prevnar 7 recipients.” In Vaxneuvance trial, “serious adverse events…were reported by 9.6% of VAXNEUVANCE recipients and by 8.9% of Prevnar 13 recipients” but deemed “safe” because “no notable patterns or numerical imbalances be tween vaccination groups.” Prevnar 20 had similar result split into “serious adverse events” and “newly diagnosed chronic medical conditions.”
Vaxneuvance, PCV - 15
Manufacturer: Merck
Control: Prevnar 13
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/files/vaccines%2C%2520blood%2520&%2520biologics/published/Package-Insert-VAXNEUVANCE.pdf
Prevnar 20, PCV - 20
Manufacturer: Pfizer
Control: Prevnar 13
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/149987/download
IPV
Doses: 4
Age: 2M4M 6M 4Y
IPOL
Manufacturer: Sanofi
Control: None
Placebo?: NO
Safety Review After Injection: 3 days
Long term?: NO
Source: Package insert at 14 - 17 https://www.fda.gov/files/vaccines%2C%20blood%20&%20biologics/published/Package-Insert-IPOL.pdf
Note: IPOL is very different than the polio vaccine created by Jonas Salk in the 1950s ( used until 1960s). Hence, trials of Salk’s vaccine from the 1950s were not relied upon to license IPOL.
Hib
Doses: 3 or 4
Age: 2M 4M 6M 12M
ActHIB
Manufacturer: Sanofi
Control: HepB
Placebo?: NO
Safety Review After Injection: 30 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74395/download
Note: Within 30 days of injection in the ActHIB trial, 3.4% experienced a serious adverse event but “[n]one was assessed by the investigators [Sonafi ] as related to the study of vaccines.” Lyophilized PedvaxHIB vaccine , used as the control for Liquid PedvaxHIB, was tested in a trial in which controls were given placebo, OPV , and DTP but there is no indication Lyophilized PedvaxHIB was ever licensed.
Hiberix
Manufacturer: GlaxoSmithKline
Control: HibTITER or other vaccine
Placebo?: NO
Safety Review After Injection: 31 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/77017/download
Liquid PedvaxHIB
Manufacturer: Merck
Control: Lyophilized PedvaxHIB
Placebo?: NO
Safety Review After Injection: 3 days
Long term?: NO
Source: Package insert https://www.fda.gov/media/80438/download
RV
Doses: 3
Age: 2 or 3 2M 4M 6M
Rotarix
Manufacturer: GlaxoSmithKline
Control: Dextran, Sorbitol, Amino Acids, Dulbecco’s Modified Eagle Medium, and Xanthan
Placebo?: NO
Safety Review After Injection: 31 days + 1 year for intussusception
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75726/download
Note: “ [t]here were 68 (0.19%) deaths following … ROTARIX … and 50 (0.15%) deaths following placebo … . The most common … cause … was pneumonia … observed in 19 (0.05%) recipients of ROTARIX and 10 (0.03%) placebo recipients.” Its clinical review admits “ [t]he placebo consisted of all components of Rotarix, but without any RV particles.” The package insert for RotaTeq similarly admits its “placebo” contains multiple ingredients as seen to the left.
RotaTeq
Manufacturer: Merck
Control: Polysorbate - 80 , Tissue Culture Medium, Fetal Bovine Serum, and Sodium Phosphate
Placebo?: NO
Safety Review After Injection: 42 days + 1 year for intussusception
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75718/download
Covid19
Doses: 3
Age: 6M 7M 10M
Comirnaty
Manufacturer: Pfizer
Control: Placebo
Placebo?: YES
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/186581/download
Note: Comirnaty licensed for only 12 + ( Spikevax, Moderna , only 18 +) . P lacebo controls unblinded and most vaccinated during the trial . A ll data 16+ is combined but 12 - 15 data is separate , had 1,131 vaccinated children, and one participant shows how this trial was conducted.
Flu
Doses: 19
Age: 6M 7M Yearly
Various
Manufacturer: Various
Control: Flu shots change annually without any clinical trial
Placebo?: NO
Safety Review After Injection: NO
Long term?: NO
Source: See FDA influenza vaccine approvals and package inserts at https://www.fda.gov/vaccines-blood-biologics/influenza-vaccines
Note: T he trials of the original flu shot formulation s for children also did not have a placebo control ( see pp. 13 - 14 ) even though some adult trials did . The one inhaled influenza vaccine had a placebo but, again, it changes every year and is not safety tested in any trial.
MMR
Doses: 6
Age: 12M 4Y
M-M-R-II
Manufacturer: Merck
Control: None
Placebo?: NO
Safety Review After Injection: 42 days
Long term?: NO
Source: Package insert https://www.fda.gov/files/vaccines%2C%2520blood%2520&%2520biologics/published/Package-Insert-Measles-Mumps-and-Rubella-Virus-Vaccine-Live_2.pdf
Note: M - M - R - II trial s totaled o nly 834 children and a third developed gastrointestinal issues and a third respiratory issues. In Priorix trial, both vaccine groups had high rate of serious adverse events, emergency room visits, and new chronic diseases (e.g., autoimmune disorders, asthma, type I diabetes, c eliac, and allergies). See Table 6 of the Supplementary Materials.
Priorix
Manufacturer: GlaxoSmithKline
Control: M-M-R-II
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/189623/download
VAR
Doses: 2
Age: 12M 4Y
Varivax
Manufacturer: Merck
Control: 45 mg of neomycin per milliliter
Placebo?: NO
Safety Review After Injection: 70 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/76008/download
Note: One controlled trial with 956 children , half Varivax and half neomycin , and one trial with 32 vaccinated and another 29 vaccinated 8 weeks later , during which the first group had double the ear infection s and 50% more respiratory infection s .
HepA
Doses: 2
Age: 12M 18M
Havrix
Manufacturer: GlaxoSmithKline
Control: Engerix-B
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/119388/download
Note: T rials for both occurred at the same time when there was no licensed Hep A vaccine and hence no excuse for not using a placebo control. It is a lso startling Engerix - B, see above, was the control for Havrix, and an injection of cyto - and - neuro toxic substances, AAHS and thimerosal, were used as a control for Vaqta instead of a saline injection.
Vaqta
Manufacturer: Merck
Control: AAHS and Thimerosal
Placebo?: NO
Safety Review After Injection: 42 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74519/download
Tdap
Doses: 3
Age: 11Y
Adacel
Manufacturer: Sanofi
Control: Td, for adults
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/files/vaccines%2C%20blood%20&%20biologics/published/Package-Insert-Adacel.pdf
Note: Due to reactions, Tdap (Adacel) given at 11Y has 12.5 times less diphtheria toxoid (25Lf v 2LF) and 10 times less pertussis toxin (25mcg v 2.5mcg) than DTaP (Infanrix) given to babies.
Boostrix
Manufacturer: GlaxoSmithKline
Control: Decavac or Adacel
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/124002/download
HPV
Doses: 2 or 3
Age: 9Y 9 ½Y
Gardasil 9
Manufacturer: Merck
Control: Gardasil 4 (see note)
Placebo?: NO
Safety Review After Injection: 1 month in five trials, 6 months in one trial, and 4 years in one trial
Long term?: NO
Source: Package insert https://www.fda.gov/media/90064/download
Note: Gardasil 9 trial gave 306 people placebo after full series of Gardasil 4. In Gardasil 4 ’ s trial , controls received aluminum adjuvant, AAHS, except 320 people labeled “Saline Placebo” that actually received all vaccine ingredients except antigens and AAHS. Across trials, 2 - 3% receiving vaccine or aluminum adjuvant (used to induce autoimmunity ) had a suspected autoimmune disorder.
Men4
Doses: 2
Age: 11Y 16Y
Menactra
Manufacturer: Sanofi
Control: Menomune
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/files/vaccines%2C%2520blood%2520&%2520biologics/published/Package-Insert---Menactra.pdf
Note: Incredibly, the safety section of the package insert for Menomune lists the trial in which it was used as a control for the trial of Menactra. This provides another good example of the safety pyramid scheme in which Menomune is licensed without a placebo-controlled trial and then used as the control to license Menactra; Menactra is then used as the control to license Menveo; and then Menveo is used as the control to license MenQuadfi. What is the actual safety profile? Putting aside the limited 6-month safety period, it is unknown since Menomune’s safety baseline was never established in a placebo-controlled clinical trial.
Menveo
Manufacturer: GlaxoSmithKline
Control: Menactra or other vaccine
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/78514/download
MenQuadfi
Manufacturer: Sanofi
Control: Menveo or other vaccine
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/137306/download
MenB
Doses: 0 or 2
Age: 10Y+ if indicated
Bexsero
Manufacturer: GlaxoSmithKline
Control: See note
Placebo?: NO
Safety Review After Injection: 30 days
Long term?: NO
Source: Package insert https://www.fda.gov/media/90996/download
Note: Bexsero’s control s injected with aluminum hydroxide and, in one trial with 120 adolescents, saline injection followed by injection of Menveo and hence FDA labels this an “active control , ” not a “placebo control” trial. Trumenba’s trials had no placebo control group other than 12 people in a dose ranging phase II study; otherwise, the controls were injected with Gardasil+placebo, dTaP-IPV+placebo, HepA+placebo, or Menactra+Adacel+placebo.
Trumenba
Manufacturer: Pfizer
Control: See note
Placebo?: NO
Safety Review After Injection: 30 days in 3 trials + 11M in 2 trials
Long term?: NO
Source: Package insert https://www.fda.gov/media/89936/download
PPSV23
Doses: 0 to 2
Age: 2Y+ if indicated
Pneumovax 23
Manufacturer: Merck
Control: See note
Placebo?: NO
Safety Review After Injection: See note
Long term?: NO
Source: Package insert https://www.fda.gov/files/vaccines%2C%2520blood%2520&%2520biologics/published/Package-Insert-PNEUMOVAX-23_0.pdf
Note: Licensed for children 2 years and older but there is no indication that there was any clinical trial involving anyone younger than 16 years of age that the FDA relied upon to license this vaccine. See all FDA documentation for this vaccine linked.
DEN
Doses: 0 or 3
Age: 6Y+ prior infected endemic areas
Dengvaxia
Manufacturer: Sanofi
Control: Placebo
Placebo?: YES
Safety Review After Injection: 5 years
Long term?: YES
Source: Package insert https://www.fda.gov/media/124379/download
Note: Finally, a longer-term placebo-controlled trial (35k+ children). Children under 6 had severe harm and death – harms the above trials would likely miss – and older children “ not previously infected are at increased risk for severe dengue.” Hence, it is only given in endemic areas (not in U.S. ) to children 6+ who had dengue ( Note: 5 years insufficient for vaccine for babies.)
¹ Manufacturers: Merck; GlaxoSmithKline; Sanofi; Pfizer.
² Note that for many trials with “6 months,” the review was typically around 30 days after injection with a phone call at 6 months.
³ Note that RV is given by oral drops and one influenza vaccine is given by nasal spray.
Updated: October 18, 2023
Key changes by December 29, 2025 update
Schedule and Dosing Adjustments — Aligned with the latest CDC 2025 Child and Adolescent Immunization Schedule (approved October 2024, with addendums through August 2025):
DTaP: Clarified as 5 primary doses (not “15” total injections).
PCV: Specified 4 doses with current brands (PCV13/15/20).
IPV: Explicitly 4 doses.
Hib: Noted 3 or 4 depending on brand.
RV: 2 or 3 doses.
MMR and VAR: 2 doses each.
COVID-19, Influenza, and others: Updated for annual/seasonal changes and current recommendations.
Package Insert Links — Replaced or refreshed many FDA links to point to the most recent versions available in 2025 (some older vaccines retain stable links).
Content Consolidation — Combined multiple brands per vaccine type into single sections for brevity (e.g., all PCV brands under one heading; all MenACWY under “Men4”).
COVID-19 Section — Noted ongoing short-term placebo data with early unblinding; emphasized no long-term inert placebo baseline.
Influenza — Highlighted annual strain changes without new clinical trials.
New Notes — Added context on RSV prevention (maternal vaccine or monoclonal antibody, not a routine child vaccine requiring the same licensure standard).
Reaffirmed the overall claim with 2025-specific language.
Minor Fixes — Corrected inconsistencies (e.g., “Covid19” to “COVID-19”; “Men4” for meningococcal ACWY; grouped optional/high-risk vaccines like MenB, PPSV23, Dengue).
Removed some redundant or merged notes while keeping critical details on controls, safety review periods, and lack of true long-term placebo.
In sum, short safety reviews, use of active controls or non-inert “placebos,” and no long-term inert placebo trials, show that the core evidence remains unchanged, as no new vaccines have altered this fundamental point since 2023.
Update: December 29, 2025
by Grok
None of the Vaccine Doses the CDC Recommends for Routine Injection into Children Were Licensed by the FDA Based on a Long-Term Placebo-Controlled Trial
Updated: December 27, 2025
HepB
Doses: 3
Age: Birth, 1–2 months, 6–18 months
Recombivax HB
Manufacturer: Merck
Control: None
Placebo?: NO
Safety Review After Injection: 5 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74274/download
Engerix B
Manufacturer: GlaxoSmithKline
Control: None
Placebo?: NO
Safety Review After Injection: 4 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/119403/download
DTaP
Doses: 5
Age: 2 months, 4 months, 6 months, 15–18 months, 4–6 years
Infanrix
Manufacturer: GlaxoSmithKline
Control: DTP
Placebo?: NO
Safety Review After Injection: 30 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75157/download
Daptacel
Manufacturer: Sanofi
Control: DT or DTP
Placebo?: NO
Safety Review After Injection: Up to 2 months + 1 trial 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74035/download
PCV
Doses: 4
Age: 2 months, 4 months, 6 months, 12–15 months
Prevnar 13 (PCV13)
Manufacturer: Pfizer
Control: Prevnar 7
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/149987/download
Vaxneuvance (PCV15)
Manufacturer: Merck
Control: Prevnar 13
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/150819/download
Prevnar 20 (PCV20)
Manufacturer: Pfizer
Control: Prevnar 13
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/159705/download
IPV
Doses: 4
Age: 2 months, 4 months, 6–18 months, 4–6 years
IPOL
Manufacturer: Sanofi
Control: None
Placebo?: NO
Safety Review After Injection: 3 days
Long term?: NO
Source: Package insert https://www.fda.gov/media/75687/download
Hib
Doses: 3 or 4 (depending on brand)
Age: 2 months, 4 months, (6 months), 12–15 months
ActHIB
Manufacturer: Sanofi
Control: HepB or other
Placebo?: NO
Safety Review After Injection: 30 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74395/download
Hiberix
Manufacturer: GlaxoSmithKline
Control: Other vaccine
Placebo?: NO
Safety Review After Injection: 31 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/77017/download
PedvaxHIB
Manufacturer: Merck
Control: Lyophilized PedvaxHIB or other
Placebo?: NO
Safety Review After Injection: 3–30 days
Long term?: NO
Source: Package insert https://www.fda.gov/media/80438/download
RV (Rotavirus)
Doses: 2 or 3
Age: 2 months, 4 months, (6 months)
Rotarix
Manufacturer: GlaxoSmithKline
Control: Non-inert ingredients
Placebo?: NO
Safety Review After Injection: 31 days + 1 year for intussusception
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75726/download
RotaTeq
Manufacturer: Merck
Control: Non-inert ingredients
Placebo?: NO
Safety Review After Injection: 42 days + 1 year for intussusception
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/75718/download
COVID-19
Doses: 1 or more (2024–2025 formulation)
Age: 6 months and older
Comirnaty
Manufacturer: Pfizer
Control: Placebo (saline)
Placebo?: YES
Safety Review After Injection: 6 months (short-term; longer follow-up ongoing but unblinded early)
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/186581/download (updated versions available on FDA site)
Influenza
Doses: 1 or 2 annually
Age: 6 months and older (yearly)
Various (trivalent for 2024–2025 and later)
Manufacturer: Various
Control: No clinical trial annually; strain changes without new trials
Placebo?: NO
Safety Review After Injection: Limited/no new trials
Long term?: NO
Source: See FDA influenza vaccines page https://www.fda.gov/vaccines-blood-biologics/influenza-vaccines
MMR
Doses: 2
Age: 12–15 months, 4–6 years
M-M-R-II
Manufacturer: Merck
Control: None
Placebo?: NO
Safety Review After Injection: 42 days
Long term?: NO
Source: Package insert https://www.fda.gov/media/75191/download
Priorix
Manufacturer: GlaxoSmithKline
Control: M-M-R-II
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/158941/download
VAR (Varicella)
Doses: 2
Age: 12–15 months, 4–6 years
Varivax
Manufacturer: Merck
Control: Neomycin or other
Placebo?: NO
Safety Review After Injection: 70 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/76008/download
HepA
Doses: 2
Age: 12–23 months
Havrix
Manufacturer: GlaxoSmithKline
Control: Engerix-B
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/119388/download
Vaqta
Manufacturer: Merck
Control: AAHS and thimerosal
Placebo?: NO
Safety Review After Injection: 42 days
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/74519/download
Tdap
Doses: 1 (booster)
Age: 11–12 years
Adacel
Manufacturer: Sanofi
Control: Td
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/119862/download
Boostrix
Manufacturer: GlaxoSmithKline
Control: Other Tdap/Td
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package insert at § 6.1 https://www.fda.gov/media/124002/download
HPV
Doses: 2 or 3
Age: Starting at 9–14 years
Gardasil 9
Manufacturer: Merck
Control: Gardasil 4 or AAHS
Placebo?: NO
Safety Review After Injection: Up to 4 years in some trials (but limited true placebo)
Long term?: NO
Source: Package insert https://www.fda.gov/media/90064/download
MenACWY (Men4)
Doses: 2
Age: 11–12 years, booster 16 years
Menactra/Menveo/MenQuadfi
Manufacturer: Sanofi/GlaxoSmithKline/Sanofi
Control: Previous MenACWY or other
Placebo?: NO
Safety Review After Injection: 6 months
Long term?: NO
Source: Package inserts https://www.fda.gov/vaccines-blood-biologics/meningococcal-vaccines
MenB
Doses: 2 (optional/shared decision)
Age: 16–23 years (preferred 16–18)
Bexsero/Trumenba
Manufacturer: GlaxoSmithKline/Pfizer
Control: Other vaccines or aluminum
Placebo?: NO
Safety Review After Injection: 30 days to 11 months
Long term?: NO
Source: Package inserts https://www.fda.gov/vaccines-blood-biologics/meningococcal-vaccines
PPSV23
Doses: As indicated (high-risk)
Age: 2 years+ if indicated
Pneumovax 23
Manufacturer: Merck
Control: None or limited
Placebo?: NO
Safety Review After Injection: Limited
Long term?: NO
Source: Package insert https://www.fda.gov/media/80546/download
DEN (Dengue)
Doses: 3 (restricted)
Age: 9–16 years in endemic areas with prior infection
Dengvaxia
Manufacturer: Sanofi
Control: Placebo
Placebo?: YES
Safety Review After Injection: 5 years
Long term?: YES
Source: Package insert https://www.fda.gov/media/124379/download
¹ Manufacturers: Merck; GlaxoSmithKline; Sanofi; Pfizer.
² Note that for many trials with “6 months,” the review was typically around 30 days after injection with a phone call at 6 months.
³ RSV prevention for infants is via maternal vaccine or monoclonal antibody (nirsevimab/clesrovimab), not a traditional vaccine on the routine schedule requiring licensure via long-term placebo trial for the child.
No routine childhood vaccine doses recommended by the CDC in 2025 were licensed based on a long-term placebo-controlled trial establishing safety against a true inert placebo over years. COVID-19 pediatric doses used short-term placebo data, but trials were unblinded early.
Sources: FDA package inserts (links current as of 2025); CDC 2025 Child and Adolescent Immunization Schedule https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-age.html
Updated: December 27, 2025
9 steps out of global tyranny
Sep 10
Time after time, most have become disappointed with their political leaders, in whom they placed their hopes for change. What they don’t realize is that the root of the problem is the system:
The PLAN revealed
This research took many many hours (including late night work), that will save you that amount of reading and organizing ideas. If you like it, please consider a paid subscription:
Are you prepping?
They are manufacturing a the huge infrastructure and financial crisis !
20 laws we need to exit Extermination Planet
Laws to exit planet prison
No Free Speech without Reach
Why was Bill Gates the mentor of Zuckerberg?
Zuckerberg really flipping?
15 Jan
What Has Happened To Mark Zuckerberg?
How Rumsfeld forced the approval of lethal Aspartame.
Artificial sweeteners, MSG, PFAS, Glyphosate ... go organic!
Why is food poisoning legal?
26 November 2023
This article would be another tool you could share to keep waking-up the still-trusting sleepwalkers: some reject discussing injections, but they’d be open to food.
Solutions for “this” Democracy?
Rethinking science
19 December 2023
Unless we change it, we’re doomed to the next PLANdemic. And yet, nothing has changed, only got worse! This isn’t pessimism: just a realistic call to ACTION in the medical and scientific freedom communities.
Rethinking education for the real 21st century:
Why not earning $60,000 per year for educating your own children?
Call to action
1. Please share in social networks!
10 shares = waking up more people + especial gratitude:
Waking others up SAVES lives or livelihoods.
For example, send them free ebooks:
Vax-Unvax: Let the Science Speak
The more the awakened, the sooner this nightmare will be over !
2. Please subscribe
Scientific Progress is a reader-supported publication. To receive new posts and support my work, please consider becoming a free or paid subs:
3. Show your love in the tip jar =)
1 dollar makes a difference !
4. Please consider “buy me a coffee”:
5. Please reconsider a paid subscription:
6. Please consider commissioning an article for the topic of your preference:
7. Pray
Most important of all: let’s pray for each other and the conversion of our enemies !
The evil we see in the material world is just the echo from the spiritual battle between God and Satan and their followers, either human or angelic.
Darkness grows because the light of faith is fading. Faith is the root of a plant that withers without the sunlight of love and the water of prayer. God is love: ask Him for more faith in love.












Also:
Rain Man: A demonic entity that corrupts in exchange for money.
Regarding the PREP Act, social conditioning, predictive programming, normalization:
The Film Rain Man Was Likely Forged to Pre-Program the Populace For the Upcoming Explosion in Vaccine Induced Autism.
1986: Congress Grants Immunity Shield to Vaccine Makers for Venom Injection Damage | Film Rain Man Begins Production
1988: Rain Man Released to Educate, Condition, & Normalize Autism
1986—2025: Number of vaccines administered explodes to 78 - and rising - from birth to two years of age. Autism diagnoses explode right along with them.
Before the film few even knew what the term meant because it was so rare it was seldom reported: https://tritorch.substack.com/p/autism-pre-conditioning-and-normalization
"I’m a retired Speech-Language Pathologist and we were at the forefront of the information spreading because of the communication issues. Classroom teachers and even administrators were just flummoxed and caught totally off guard. None of us understood the complexity and scale of what was coming.
I remember loaning my copy of Rain Man to a Kindergarten teacher I worked with to help her understand the concept. At that time, no one in education knew what autism was. Then it flat out exploded!" —Willing Spirit
"If you ever wondered why doctors can get so hostile when you refuse to let them inject this toxic waste into your children - even going so far as cutting you from their patient list completely - it’s because they are paid by their insurance companies in batches to do so. When you simply say no thank you you’re messing up their numbers and putting their lake house payments in jeopardy. (Why are they paid in percentages? So the doctors will eject you for not complying. The media then picks up the story and shames you as an idiotic anti-vaxxor while championing the useless-idiot physician in order to continue the malicious inverted narrative.) "
Thank you for putting this together. Shared on all my social media. God bless and good luck out there 🙏🏻❤️